Chronic Lymphocytic Leukemia (CLL)
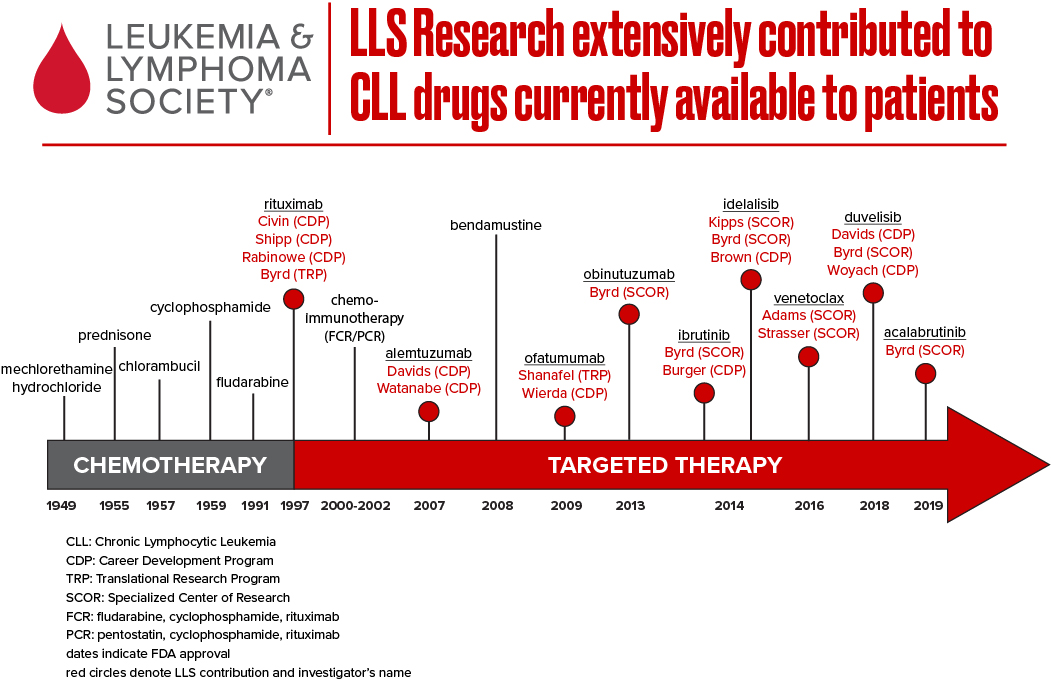
Over the past 10 years, The Leukemia & Lymphoma Society (LLS) has invested more than $52 million to accelerate pioneering research in chronic lymphocytic leukemia (CLL), a blood cancer characterized by the development of too many white blood cells called lymphocytes. Our investment has unlocked new insights on CLL disease mechanisms and advanced new therapies to improve outcomes and care for patients. Despite its name, Chronic Lymphocytic Leukemia is actually a type of non-Hodgkin lymphoma.
Are you a Patient or Caregiver? Click here for our free informational booklet on CLL.
Changing the CLL treatment landscape
The therapeutic landscape for chronic lymphocytic leukemia (CLL), the most common adult leukemia in the U.S., has evolved significantly in the past decade. Next-generation sequencing has expanded our knowledge of the molecular underpinnings of the disease, ushering in promising precision medicine approaches. LLS has been at the forefront of CLL treatment innovation and continues to invest in cutting edge research to find cures.
Today, the CLL treatment armamentarium includes “classical” cytotoxic chemotherapy agents, such as purine analogs and alkylating agents; monoclonal antibodies, including rituximab (Rituxan®), obinutuzumab (Gazyva®), ofatumumab (Arzerra®), inhibitors of BCR signaling, such as BTK inhibitors (e.g. ibrutinib, acalabrutinib and zanabrutinb) and P13K inhibitors (e.g. idelalisib and duvelisib); and an inhibitor of the machinery that control cell death (e.g. venetoclax). CLL is extremely similar to small lymphocytic lymphoma (SLL) – accordingly, many oncologists treat CLL and SLL as the same disease with different presentations.
The role of precision medicine in CLL treatment
Despite recent progress, CLL remains incurable for most patients. Only about 20 percent of previously untreated patients will achieve the deepest possible remission with the conventional chemoimmunotherapy regimen: fludarabine, cyclophosphamide and rituximab (FCR). Remission rates are even lower in patients whose CLL has relapsed. What’s more, while one form of the disease is slow growing and can remain stable without treatment for years, another form is aggressive and requires more immediate intervention.
Driven by recent advancements in genomics, researchers know significantly more about the complex heterogeneity of CLL today and are applying a variety of clinical, biological and genetic parameters to evaluate cases – opening the door to more tailor-made therapeutic approaches.
Factors such as chromosomal alterations, somatic mutations, antigenic stimulation and changes in epigenetics (controls of gene expression) play a role in CLL disease development. Certain abnormalities are associated with different disease prognoses – for example, the chromosomal alternations 11q deletion and 17p deletion, found in 18 percent and 7 percent of patients respectively, are associated with a particularly poor prognosis.
Targeted therapy – a cornerstone of precision medicine – is transforming CLL treatment by homing in on specific molecular or genetic drivers of the disease. LLS is laser-focused on advancing novel therapies and optimizing the best combination and sequence of treatments so more patients with CLL can experience long-term disease control and improved quality of life.
LLS is driving CLL treatment innovation
LLS has helped advance the latest approved therapies for CLL/SLL, including ibrutinib (Imbruvica®), venetoclax (Venclexta®) and idelalisib (Zydelig®) – three game changing oral therapies that have demonstrated remarkable efficacy for patients – along with the immunotherapy obinutuzumab. Over the past 15 years, LLS has invested more than $30 million into the discovery and advancement of ibrutinib and venetoclax alone.
Venetoclax, first approved by the U.S. Food and Drug Administration (FDA) in 2016 for relapsed or refractory CLL/SLL patients with 17p deletion, marked a significant advance for a very high-risk group of patients. Since 2002, LLS has supported the visionary work of Jerry Adams, Ph.D., Andreas Strasser, Ph.D., and their team at the Walter and Eliza Hall Institute of Medical Research in Australia through our prestigious Specialized Center of Research (SCOR) grant program. Andrew Roberts, M.D., M.S., Ph.D., a member of Dr. Adams’ SCOR team, was one of the clinicians who led studies of the pivotal Phase 2 clinical trial of venetoclax upon which the FDA based its approval. In 2018, the FDA expanded the approval of venetoclax for relapsed or refractory CLL/SLL patients, with or without the 17p deletion, alone or in combination with rituximab.
Pursuing cures for CLL
We believe cures for CLL are on the horizon. To this end, LLS is boldly pursuing research in the following areas:
- Optimizing chemo-free combinations: ibrutinib/rituximab; venetoclax/obinutuzumab (Gazyva), or ibrutinb/venetoclax
- Advancing understanding of disease development, progression and relapse. LLS funding is helping researchers pinpoint tumor cell and microenvironment (which surrounds the tumor cells) vulnerabilities.
- Overcoming blocks in the cell death pathway. LLS-funded researchers are studying the cell death pathway, with the goal of optimizing venetoclax or developing new therapies to address resistance. Andreas Strasser, PhD, MSC, FAA, (Walter and Eliza Hall Institute of Medical Research), is leading an ambitious project centered on boosting apoptosis for CLL and other blood cancers. Dr. Strasser and his team are studying venetoclax in combination with other drugs, such as ibrutinib, to deliver a one-two punch to CLL cells. His team has already identified a novel mechanism of resistance to venetoclax that has immediate therapeutic implications.
- Advancing novel and personalized treatment regimens. Matthew Davids, M.D., (Dana-Farber Cancer Institute), is conducting clinical trials to optimize ibrutinib in combination with chemotherapy or immunotherapy. Complementing the clinical trials are laboratory studies to evaluate treatment effectiveness, including the use of new technology to sequence tumor DNA. The ultimate goal is to help oncologists test patients prior to starting treatment to understand which treatment will likely be the most effective for that particular patient.
- Developing novel immunotherapies. With support from LLS, researchers are analyzing the immune status of CLL cells and the microenvironment to pave the way for new immunotherapeutic approaches in CLL, including novel antibodies, CAR T-cell immunotherapy and checkpoint inhibitors.
- Identifying, validating and developing therapies for novel disease targets. Emerging targets for CLL include the SYK/JAK pathway, FCRL1, Notch pathway and ROR1, among others. Advancing therapies that attack these specific targets is key, particularly for patients with relapsed/refractory disease.
Although new therapies will extend the life of some CLL patients, perhaps even achieve cures (where therapy can be stopped because the disease has been eradicated), there is an unprecedented opportunity to understand the basis of resistance to new therapy. This will lead to new alternative therapies that pave the way to cures for all CLL patients.
Photograph by Gabriel Caponetti, distributed under a CC BY-SA 3.0 license